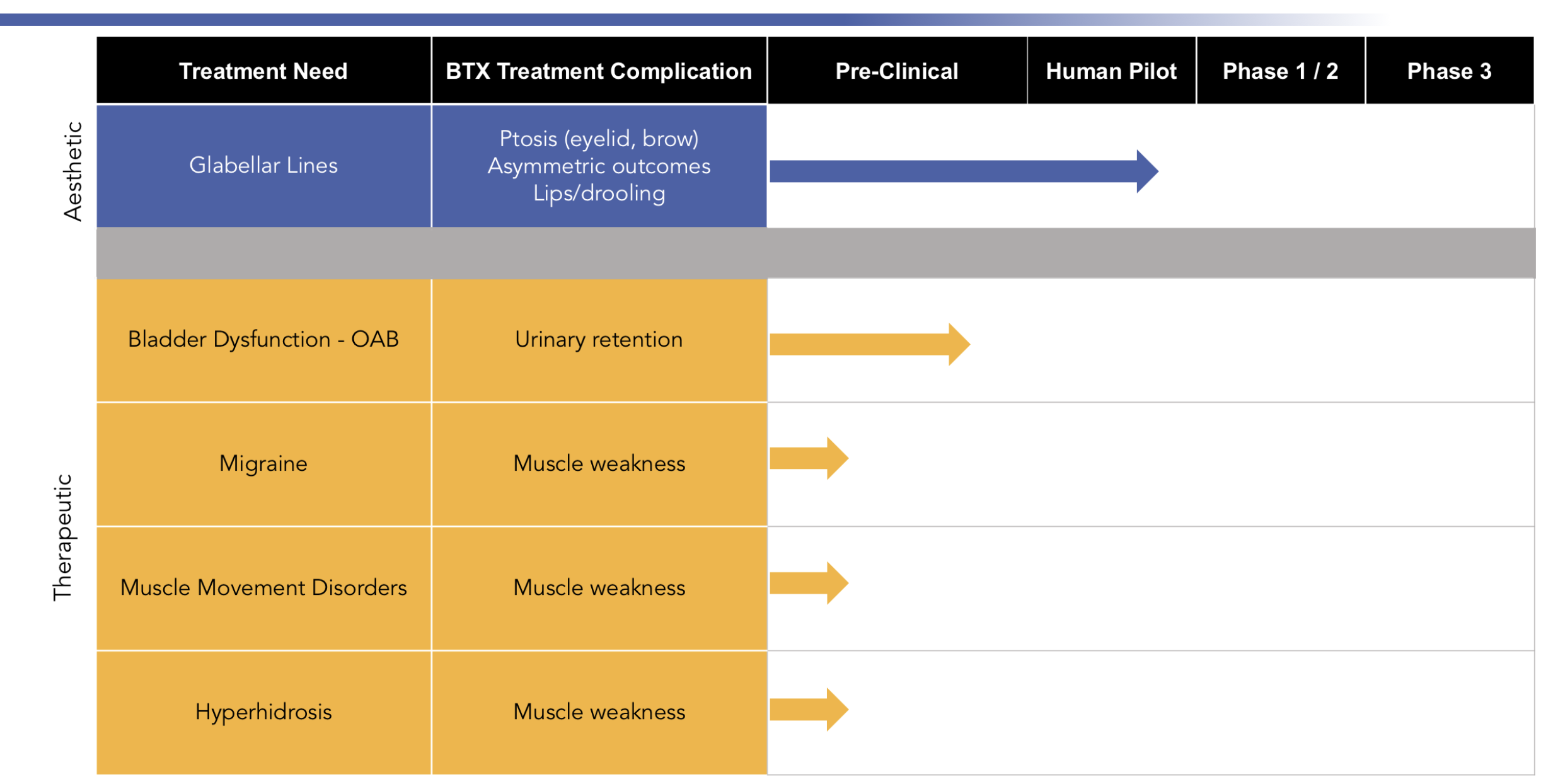
ReViVox® Pipeline
Botulinum toxin type A was the most common non-invasive cosmetic procedure of 2018, with 7.4 million procedures taking place. Approximately 44% of botulinum revenue is generated from aesthetic use with the remaining market focused on medical therapies such as migraine, overactive bladder, muscle movement conditions, hyperhidrosis and others.With growing indications for botulinum neurotoxin, the market was valued at $4.5 billion in 2017 and is projected to reach $8.7 billion by 2026.
ReViVox®, DelNova’s lead product candidate, may be effective as a rescue drug for use in any aesthetic or medical procedure which results in spread of toxin to nearby muscles, causing unintended paralysis.
ReViVox® Pipeline