
Aesthetic
Off-target muscle paralysis after botulinum toxin injections offer a large market where there is currently no approved treatment. Botulinum toxin type A was the most common non-invasive cosmetic procedure of 2018, with 7.4 million procedures taking place.
While botulinum toxin administration is popular and safe, some adverse events occur. Laws vary by state, though generally Botox™ Cosmetic or other botulinum toxin A may be administered by any medical professional (i.e. nurses, dentists, dental hygienist or aestheticians after completing an online Certification Course) Allergan, the maker of Botox™, revealed in a multicenter post- marketing FDA study a 4.6% incidence of ptosis (12 out of 263)–many of these complications were a result of an inexperienced injector (Ref. Clin M. “Management of Ptosis” J Clin Aesthet Dermatol. 2016 Dec; 9(12)).
ReViVox® is being developed as rescue product that could potentially serve this growing market and accelerate its growth.
UNINTENDED MUSCLE PARALYSIS: CONSEQUENCES
- Stroke-Like Appearance
- Droopy Eye (Ptosis)
- “Spock” Brow
- Frozen Face
- Misaligned or Puffy Lips
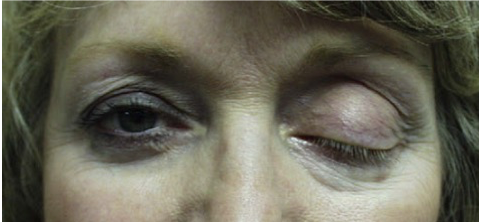
Botox™ or other botulinum toxin Type A products may cause temporary, undesirable side effects lasting weeks to months. There is no remedy other than to wait for the toxin to wear off. There are no competitors.


